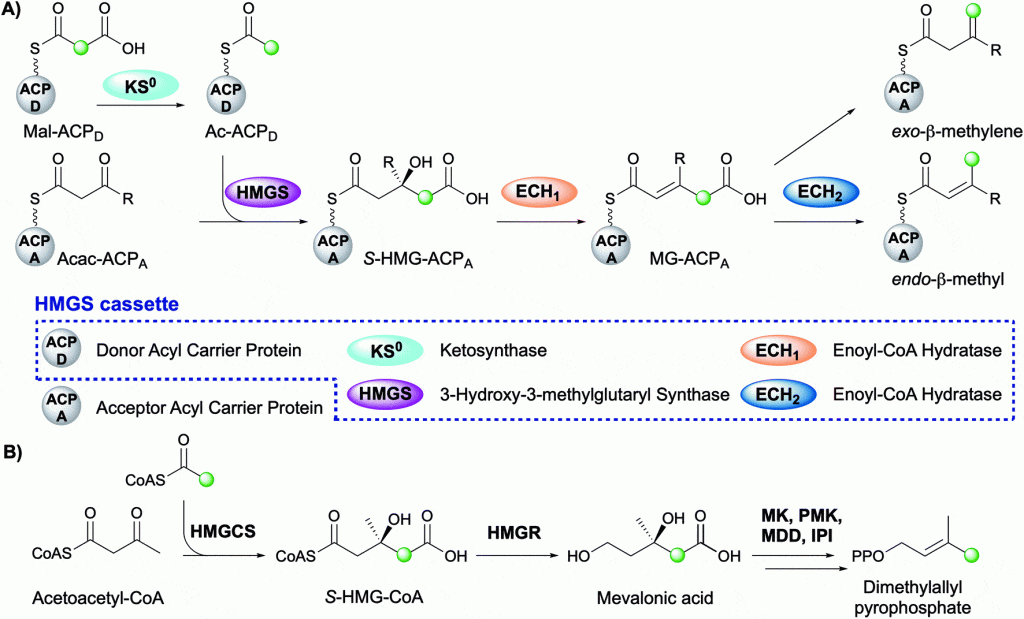
The Crump group is pleased to announce the publication of a recent review within Natural Product Reports. This review summarises the diversity of beta-branch incorporation within polyketide synthases and the mechanistic details of each catalytic step. The overall transformation is catalysed by a multi-protein 3-hydroxy-3-methylglutaryl synthase (HMGS) cassette and is reminiscent of the mevalonate pathway in terpene biosynthesis. The application of HMGS in data mining, additional beta-branching mechanisms and current knowledge of the role of beta-branches in this important class of biologically active natural products is also discussed.
Biosynthesis
Recent Paper: A Priming Cassette Generates Hydroxylated Acyl Starter units in Mupirocin and Thiomarinol Biosynthesis.
The Crump group is pleased to announce the publication of recent work in ACS Chemical Biology Journal. This work was completed as a collaboration between the Departments of Chemistry and Biochemistry at the University of Bristol, showing the need for frequent collaboration in the field of biosynthesis of natural products.
This work utilises a plethora of laboratory techniques to fully characterise the formation of a 3-carbon alcohol intermediate in the biosynthesis of the polyketide antibiotic mupirocin and homologous 4-carbon alcohol intermediate in the biosynthesis of thiomarinol.
This is exciting work and provides a solid contribution to the biosynthetic community, well done to all involed in the publication of this work.
A link to the paper can be found at the following link: https://doi.org/10.1021/acschembio.9b00969